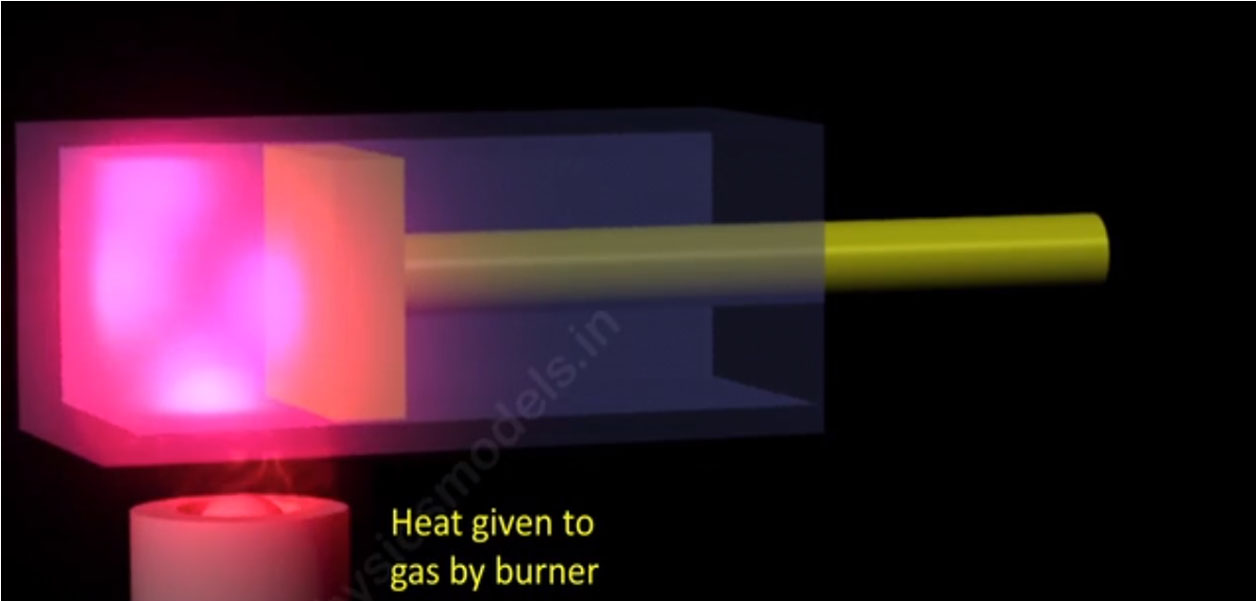
Image shows a gas contained inside a chamber, with its volume ‘V’ decreased and increased by a Piston. The Piston moving to & fro, changes the volume and associated Temperature and pressure , all 3 things being inter-related
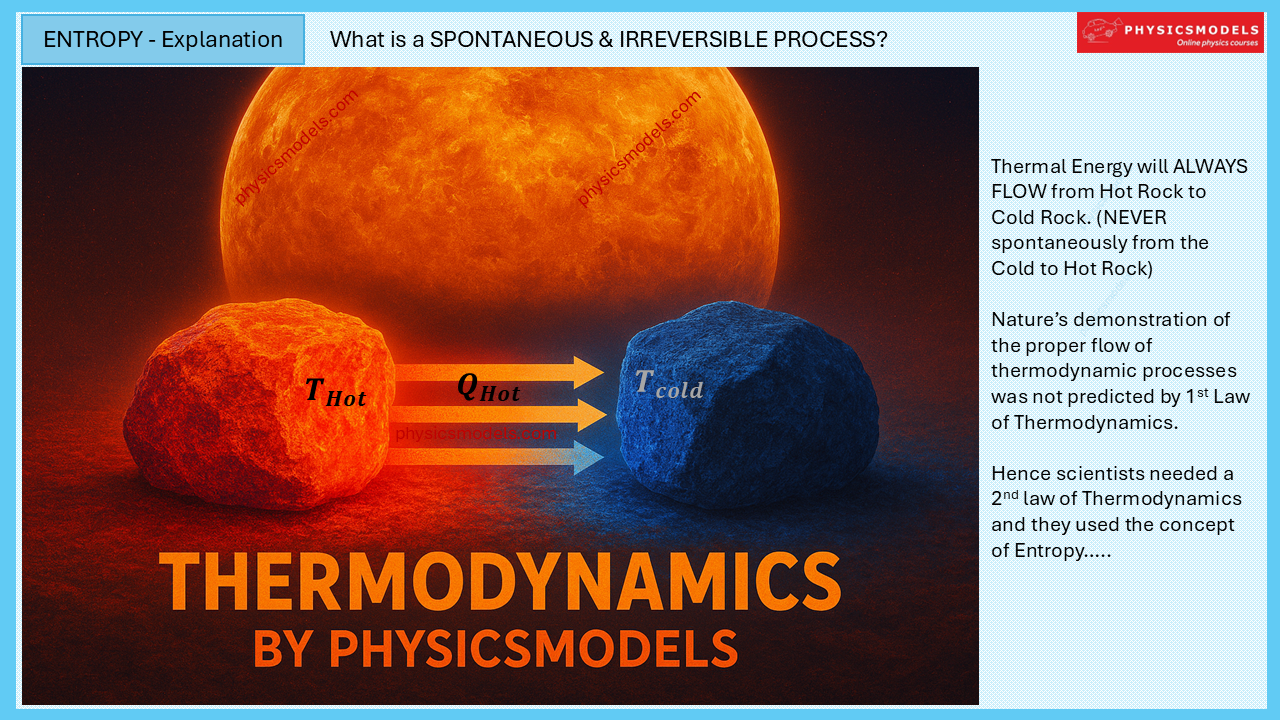
See another important aspect of Thermodynamics, the DIRECTION of thermodynamic processes. Heat Energy will only flow from Hot Body to Cold Body, not the other way round. This concept of Nature is critical to understand ENTROPY and 2nd law of Thermodynamics
Thus, Thermodynamics becomes fundamental to the working of our entire Universe, not only for the Earth.
Some of the well known natural processes of thermodynamics are given below, only for us appreciate the vastness of this subject :
- The thermal energy of the sun’s nuclear fusion and fission beaming down on the earth’s surface
- the rise in temperature of the earth’s surface and the oceans
- the rising hot air currents
- the blowing of the breeze , various winds across seas and continents
- the phenomenon of Tornadoes, Cyclones, Typhoons due to hot rising air currents, in some cases whirlpool formation
- Rigth at the other end, the extremely hot temperatues inside the earth’s crust, where even iron is in molten form, leading to molten lava coming out in Volcanic eruptions ……
Above image shows MASS as well as HEAT ENERGY coming out of a volcano.
Near the oceans, the air currents circulate constantly. Warm air rises up from the hotter ground. This forms rain clouds along with ater vapur rising from the sea. This forms rain clouds which later give us precious rain.
Meanwhile, the cold air from other neighbouring regions flows into the space vacated by the hot air . The thermodynamic cycle continues for centuries.
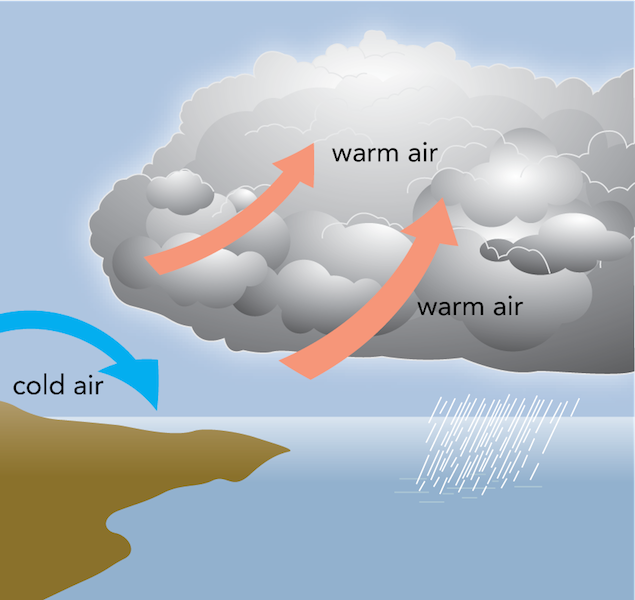
image courtesy , read more at: https://manoa.hawaii.edu/exploringourfluidearth/physical/atmospheric-effects/wind-formation
It’s amazing that Thermodynamics is always present in NATURE , and in the entire Universe. It does’nt stop, it is constantly happening everywhere , even as we speak…….
The topics covered in this course are:
1) First Law of Thermodynamics (drawbacks leading to the Second Law)
2) Work Done by a Gas
3) Isobaric , Isothermal and Adiabatic expansion : equation derivation
4) Petrol /Gasolene based Internal Combustion engine (passenger car) – a look
5) Diesel engine (passenger car) – a look
6) Entropy – The shortcoming of 1st Law, Irreversible processes, and the Second Law of Thermodynamics
7) Carnot’s Thoerem, Carnot engine, Efficiency derivation
8) Solved Problems
Course Features
- Lectures 11
- Quiz 0
- Duration Lifetime access
- Skill level All levels
- Language English
- Students 100
- Certificate No
- Assessments Yes
- 1 Section
- 11 Lessons
- Lifetime
- Laws of thermodynamics11
- 1.1STP versus NTP for a gas, and Volume of 1 mole of an ideal gas
- 1.21st Law of thermodynamics and its shortcomings
- 1.3Isothermal Process
- 1.4Work done by a gas
- 1.5Petrol engine
- 1.6Diesel engine
- 1.7Entropy – explanation, drawbacks of 1st Law, the need for 2nd law, Spontaneous and Irreversible processes
- 1.82nd Law of thermodynamics
- 1.9Heat Engines
- 1.10Carnot’s Thoerem, Carnot engine, Efficiency derivation
- 1.11Solved problems