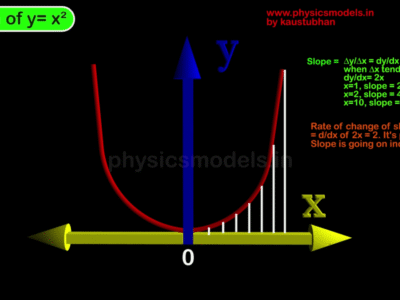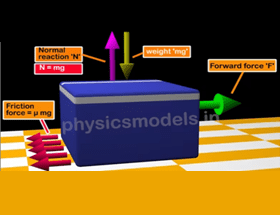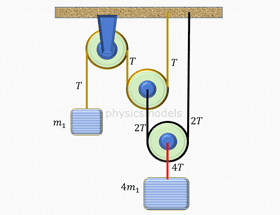
This course explains in detail about behaviour of molecules in a gas. Animations and images are included to simulate in slow motion, the motions of molecules inside a gas. The understanding of collisions of molecules against walls of a container helps in understanding gas pressure being a result of many such collisions.
Topics covered are:
1) Kinetic Theory of Gases : it’s purpose and assumptions behind it
2) What is an Ideal Gas ? – definition
3) Assumptions behind the Kinetic Theory
4) Pressure and RMS speed : for an ideal gas
5) Kinetic Theory: interpretation of temperature concept, Equations for KE and Temperature in terms of RMS speed
6) Brownian Motion
7) Vapor and Critical Temperature of a gas , Isotherms
8) Evaporation and Kinetic Energy of molecules
9) Dew Point :- Air temperature, and Saturation Vapor Pressure
10) Solved problems
Course Features
- Lectures 13
- Quiz 1
- Duration Lifetime access
- Skill level All levels
- Language English
- Students 152
- Certificate No
- Assessments Yes
- 1 Section
- 13 Lessons
- Lifetime
- Kinetic theory of gases14
- 1.1The purpose of Kinetic Theory of gases, assumptions
- 1.2The Ideal Gas Equation
- 1.3Assumptions behind Kinetic Theory
- 1.4Pressure and rms speed – Ideal Gas
- 1.5Kinetic Theory – Interpretation of temperature
- 1.6Brownian Motion
- 1.7Vapour and critical temperature of gas
- 1.8Evaporation and kinetic energy of molecules
- 1.9Dew point
- 1.10Kinetic theory of gases-Solved problems
- 1.11Kinetic theory of gases- HC Verma Solved problem-1
- 1.12Kinetic theory of gases-HC Verma Solved problem-2
- 1.13Kinetic theory of gases-HC Verma Solved problem-3
- 1.14Kinetic Theory of gases-Theory test58 Minutes16 Questions